Gas laws belong to the fundamentals of thermodynamics and are dealt with in every training course on thermodynamics.
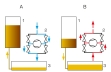
The WL 102 experimental unit enables two changes of state to be studied experimentally: isothermal change of state, also known as the Boyle-Mariotte law, and isochoric change of state, which occurs at constant volume. Transparent tanks enable the change of state to be observed. Air is used as the test gas.
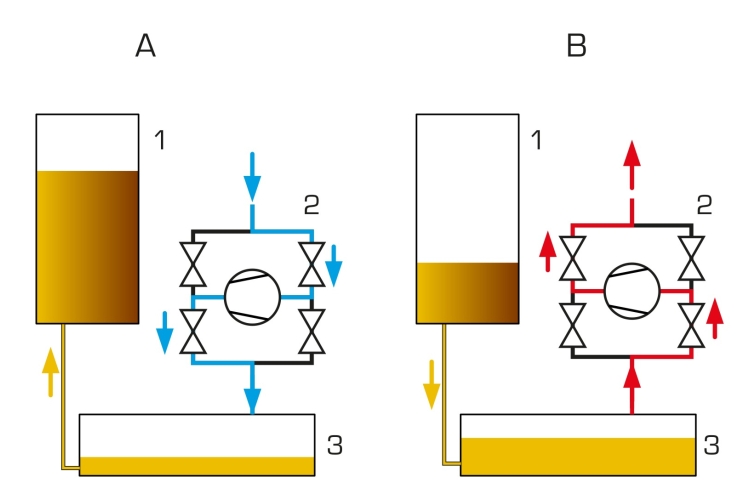
In the first tank, positioned on the left, the hermetically enclosed air volume is reduced or increased using a compressor and hydraulic oil. This results in an isothermal change of state. The compressor can also operate as a vacuum pump. If the changes occur slowly, the change of state takes place at an almost constant temperature.
In the second tank, positioned on the right, the temperature of the test gas is increased by a controlled electric heater and the resulting pressure rise is measured. The volume of the enclosed gas remains constant.
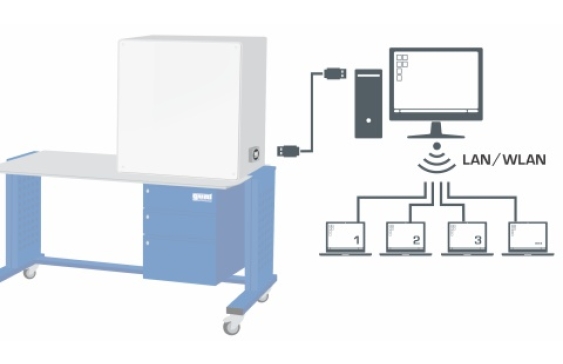
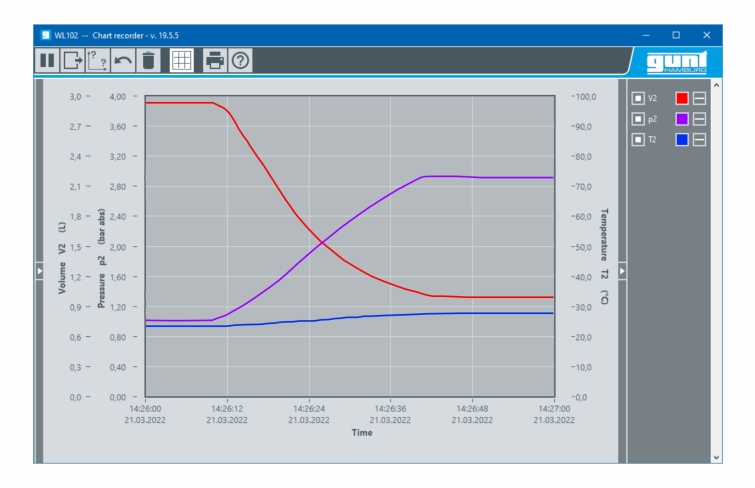
Temperatures, pressures and volume are measured electronically, digitally displayed and transferred to a PC for processing.