Crystallisation enables dissolved substances from solutions to be transformed into a solid and separated.
This trainer has been developed in cooperation with the Chair of the Thermal Process Technology at the Martin-Luther University, Halle-Wittenberg (Prof. Dr. Ulrich).
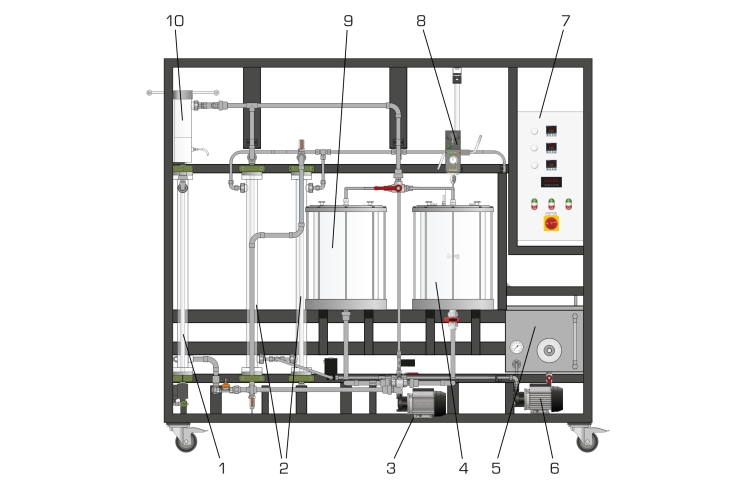
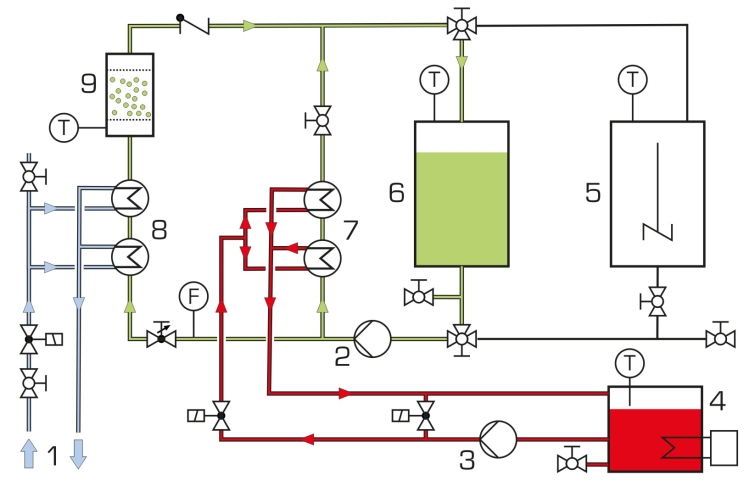
A pump delivers a saturated potassium sulphate solution in a circuit with a tank. To prevent premature crystallisation, the solution is heated above saturation temperature using a heating circuit. Both circuits are connected by two heat exchangers. A small amount of this undersaturated solution is fed through the crystallisation cell as a bypass. To crystallise this part of the solution, it is cooled by cooling water using two heat exchangers. Reducing the temperature converts the solution into an oversaturated, metastable state.
The crystallisation cell is a tube fitted with porous filter media at both the inlet and outlet. The removable cell can be opened to allow the addition of seed crystals. The porous filter media are selected in a way that the crystals can’t escape from the cell. The flow conditions cause a fluidised bed in the cell. The dissolved potassium sulphate crystallises out of the metastable solution at the seed crystals. The crystals grow. The growth rate can be determined by weighing the crystals before and after the experiment and by measurement of time.
A stirred tank with heat exchanger is available to prepare a saturated potassium sulphate solution. The temperatures in the two tanks and the temperature required in the bypass for crystallisation are recorded and controlled using sensors.
A drying chamber, a balance, a screening machine and a microscope are recommended for evaluating the experiments. Potassium sulphate is not included.