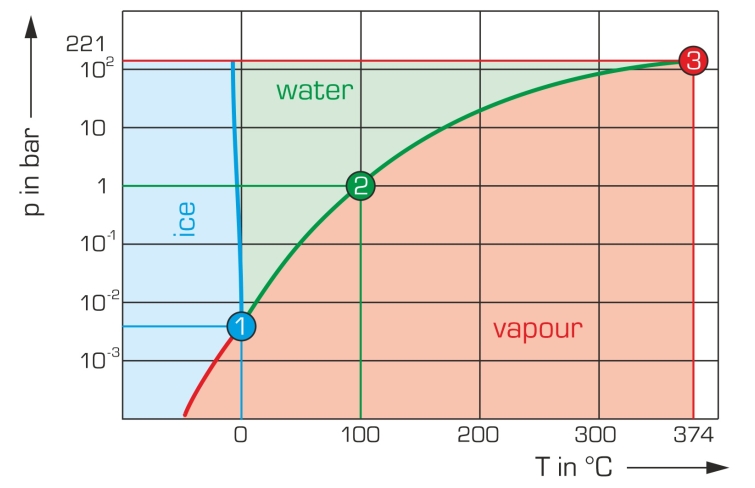
In a closed system filled with fluid, a thermodynamic equilibrium sets in between the fluid and its vaporised phase. The prevailing pressure is called vapour pressure. It is substance-specific and temperature-dependent.
When a fluid is heated in a closed tank, the pressure increases as the temperature rises. Theoretically, the pressure increase is possible up to the critical point at which the densities of the fluid and gaseous phases are equal. Fluid and vapour are then no longer distinguishable from each other. This knowledge is applied in practice in process technology for freeze drying or pressure cooking.
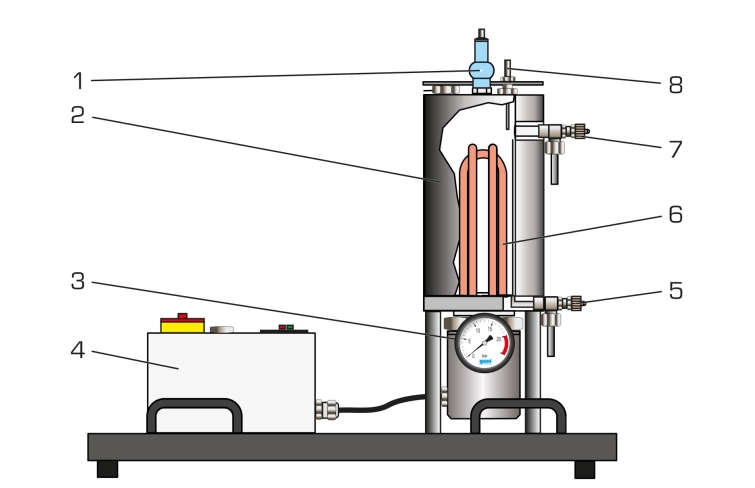
The WL 204 experimental unit can be used to demonstrate the relationship between the pressure and temperature of water in a straightforward manner. Temperatures of up to 200°C are possible for recording the vapour pressure curve. The temperature and pressure can be continuously monitored via a digital temperature display and a Bourdon tube pressure gauge.
A temperature limiter and pressure relief valve are fitted as safety devices and protect the system against overpressure.