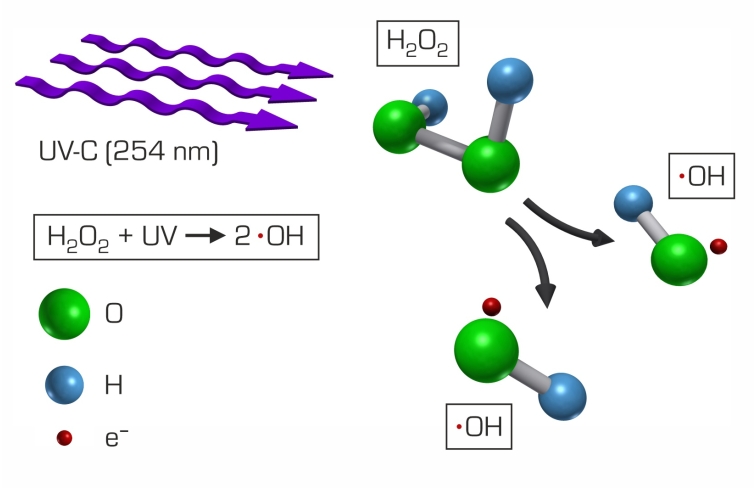
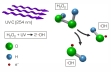
In water treatment oxidation processes are used to remove organic substances which are not biodegradable. If the oxidation is by hydroxyl radicals (OH radicals) it is called “advanced oxidation”. A common method for forming hydroxyl radicals is the irradiation of hydrogen peroxide with UV light. CE 584 demonstrates this process using a discontinuous falling film reactor.
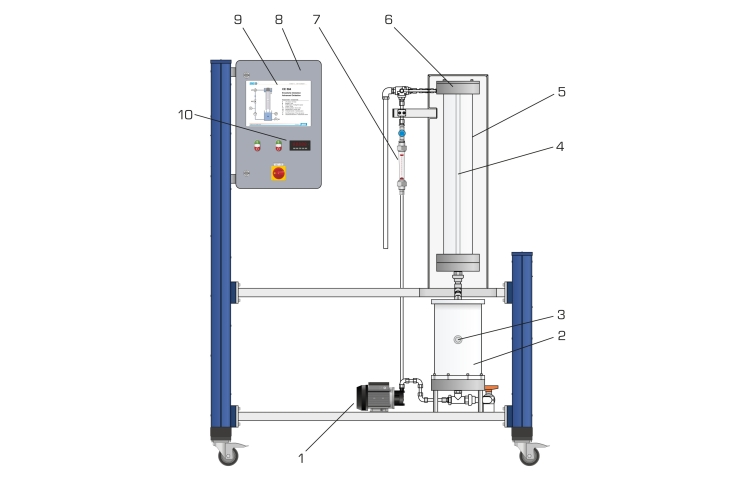
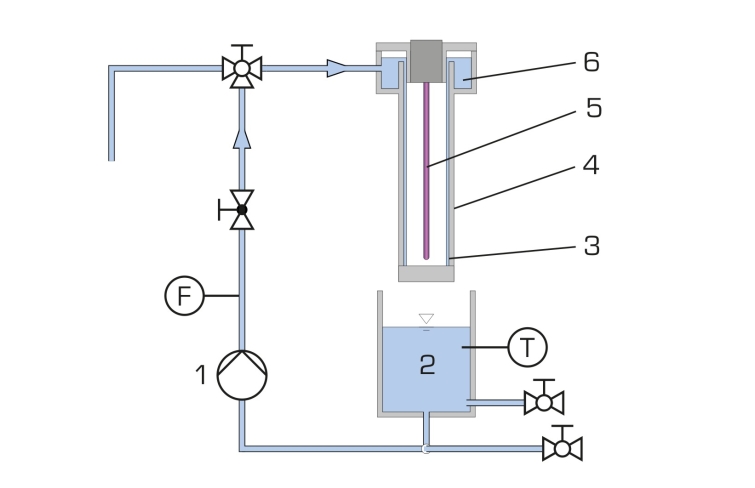
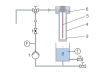
The falling film reactor consists of a transparent tube which is open at the bottom. At the top of the tube there is a circular channel. Using a pump the raw water enriched with hydrogen peroxide is transported from a tank into the channel. From here the water flows as a thin falling film along the inside wall of the tube back into the tank. This creates a closed water circuit. At the centre of the tube there is a UV lamp. By irradiation of the falling raw water with UV light hydroxyl radicals form from the hydrogen peroxide molecules. The hydroxyl radicals oxidate the organic non-biodegradable substances in the raw water. As protection against the radiation the UV lamp is fitted with a protective tube.
The flow rate and temperature of the water are continuously measured. The temperature is indicated digitally in the switch cabinet. Samples can be taken at the tank.
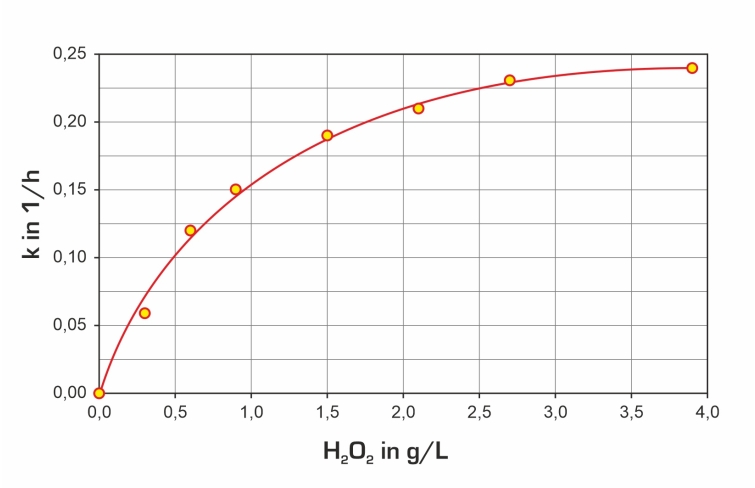
E.g. triethylene glycol dimethyl ether can be used to produce the raw water. Analysis technology is required to evaluate the experiments.